Ion Exchange:
The ion exchange process percolates water through bead-like spherical resin materials (ion-exchange resins). Ions in the water are exchanged for other ions fixed to the beads. The two most common ion-exchange methods are softening and deionization.
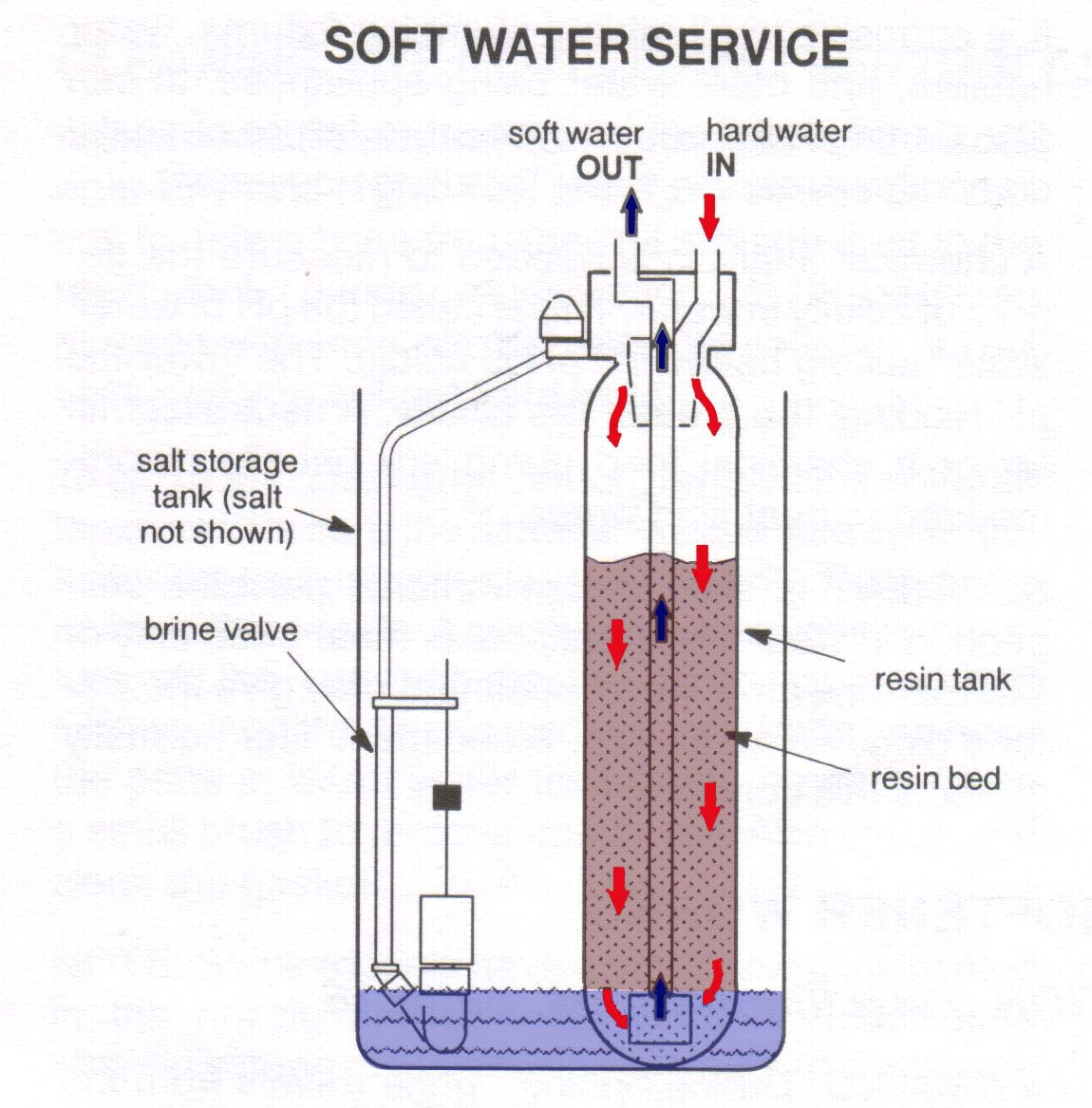
Softening is used primarily as a pretreatment method to reduce water hardness prior to reverse osmosis (RO) processing. The softeners contain beads that exchange two sodium ions for every calcium or magnesium ion removed from the “softened” water.
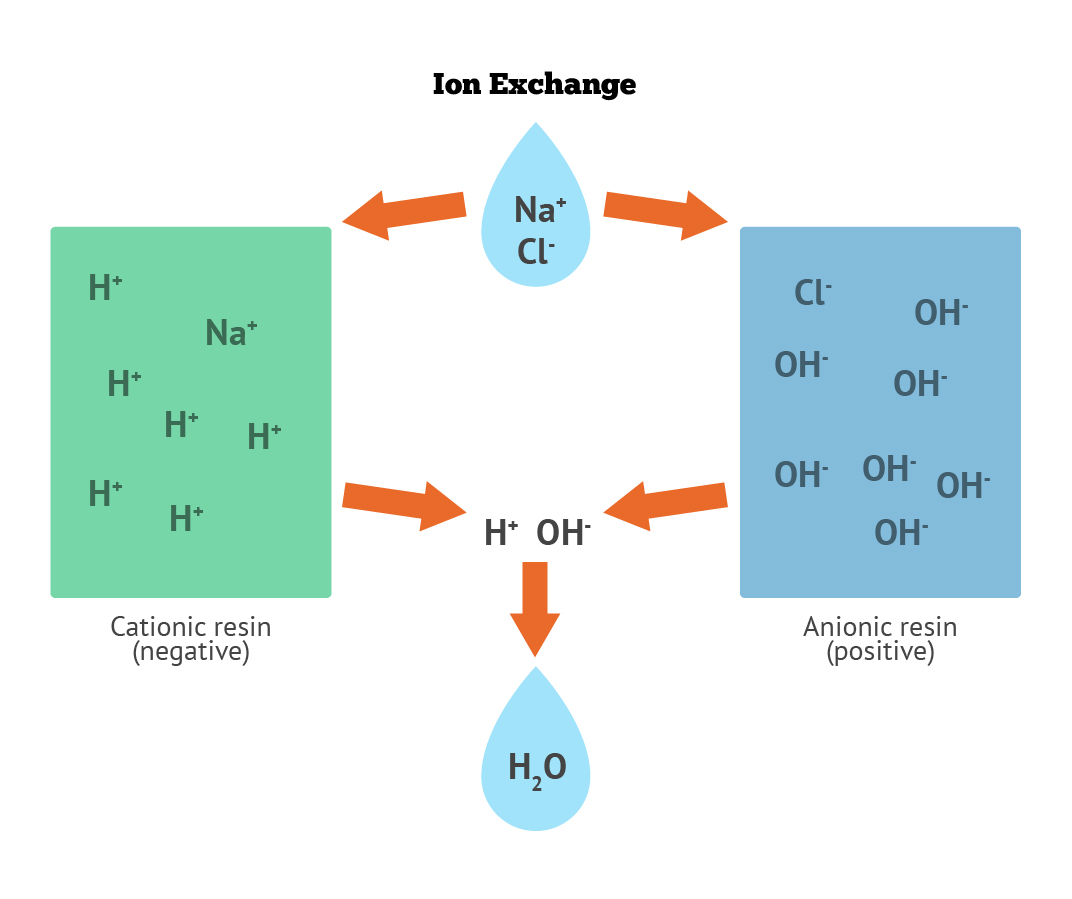
Deionization (DI) beads exchange either hydrogen ions for cations or hydroxyl ions for anions. The cation exchange resins, made of styrene and divinyl benzene containing sulfonic acid groups, will exchange a hydrogen ion for any cations they encounter (e.g., Na+, Ca++, Al+++). Similarly, the anion exchange resins, made of styrene and containing quaternary ammonium groups, will exchange a hydroxyl ion for any anions (e.g., Cl-). The hydrogen ion from the cation exchanger unites with the hydroxyl ion of the anion exchanger to form pure water.
Water Softening Plant:
Water softening is the removal of calcium, magnesium, and certain other metal cations in hard water.The resulting soft water is more compatible with soap and extends the lifetime of plumbing. Water softening is usually achieved using lime softening or ion-exchange resins.
Demineralization Water Treatment:
Demineralization is the process of removing mineral salts from water by using the ion exchange process.
Demineralized water also known as Deionized water, water that has had its mineral ions removed. Mineral ions such as cations of sodium, calcium, iron, copper, etc and anions such as chloride, sulphate, nitrate, etc are common ions present in water. Deionization is a physical process which uses specially-manufactured ion exchange resins which provides ion exchange site for the replacement of the mineral salts in water with water forming H+ and OH- ions. Because the majority of water impurities are dissolved salts, deionization produces a high purity water that is generally similar to distilled water, and this process is quick and without scale buildup.
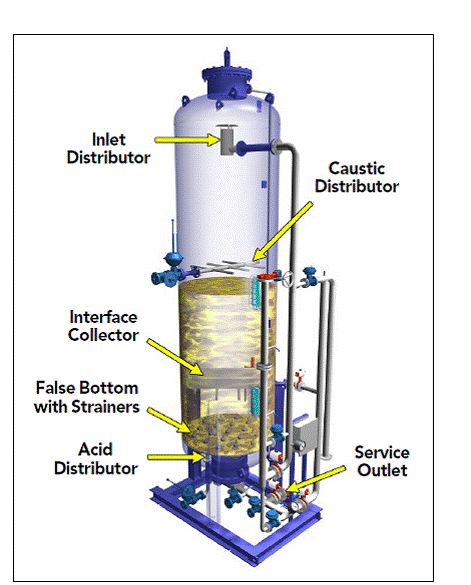
De-mineralization technology is the proven process for treatment of water. A DM Water System produces mineral free water by operating on the principles of ion exchange, Degasification, and polishing. Demineralized Water System finds wide application in the field of steam, power, process, and cooling.
Degasser Tower:
Degasser is an integral part of any demineralization plant, where it is generally placed between cation and anion exchanges and removes Carbon Dioxide, which is generated by dissociation of carbonic acid at cation outlet water. In this Degassing processes, Degasser Tower is utilized, which is made from either FRP or Mild Steel with rubber lined or epoxy coating. Low air pressure is generated at the bottom of the tower that drives out CO2 and the degassed water is collected in a sump beneath the tower.
Mix Bed Polisher:
Usually a mixed bed polishing unit contains strong acid Cation (SAC) resin and strong base Anion (SBA) resins combined to a ratio of approximately 40% Cation resin to 60% Anion resin. (All resin volumes are dependant upon the incoming water analysis and the required quantity of treated water).
Mixed bed units deliver an excellent treated water quality, but are complicated to regenerate, as the resins must first be separated by back washing before regeneration. Additionally, they require large amounts of chemicals, and the hydraulic conditions for regeneration are not optimal. Therefore, mixed beds are usually only used to treat pre-demineralised water, when the service run is long.